Stroke Registry
Overview
Stroke registration is a process of continuing systematic collection of data on the occurrence and characteristics of stroke events in persons of defined population and those who attend hospitals. The National Stroke Registry Programme has been established to generate reliable data on stroke epidemiology and patters of care. This information is crucial for the ongoing efforts of improving stroke care in the country.
A Stroke registry aims at:
- collection of data on Stroke events
- Transmission and Storage
- Analysis & interpretation
- Periodic report publication and dissemination
Types of Registries of the NSRP:
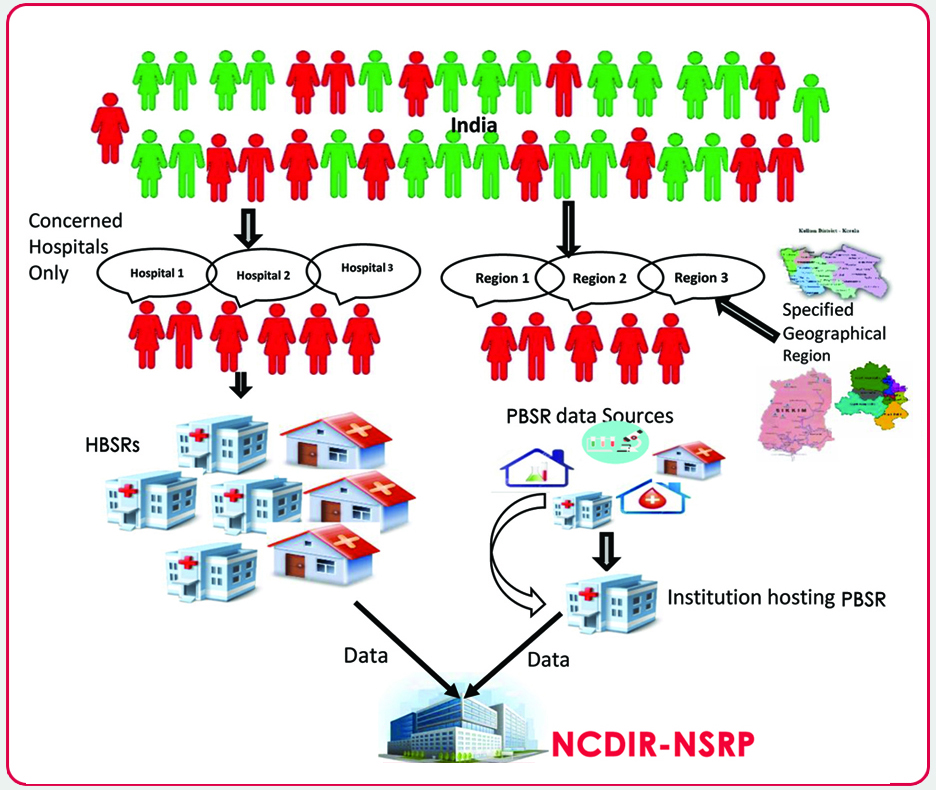
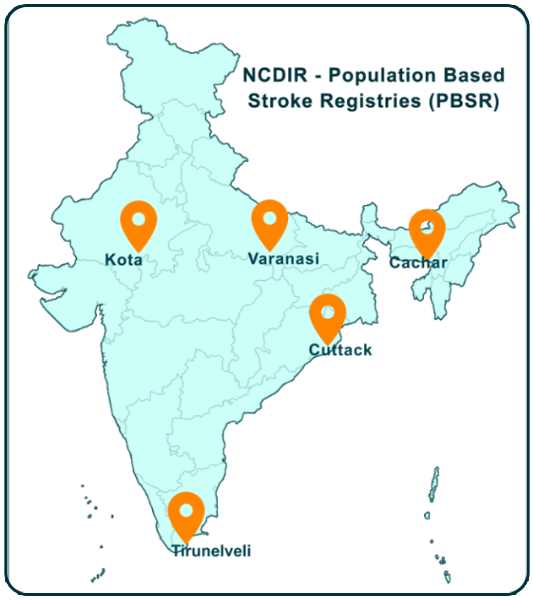
- Population based Stroke Registry (PBSR)
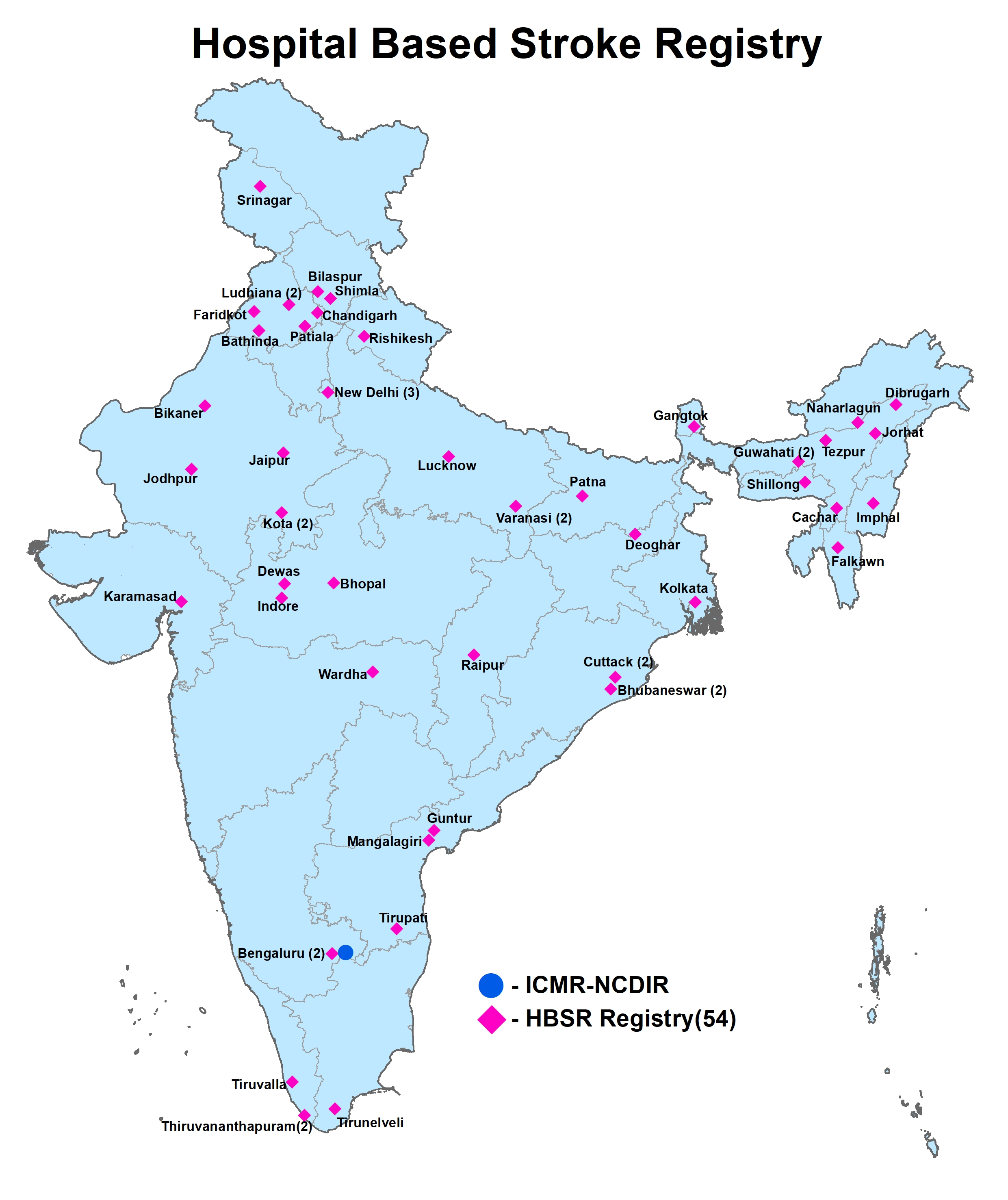
- Hospital based Stroke Registry (HBSR)
Benefits of Stroke Registration
- Develop national database on stroke burden and pattern of care and survival
- Generate evidence on patterns of stroke and access to stroke care in different treatment settings
- Generate evidence to improve preventive, curative, rehabilitative services for stroke from primary to tertiary level of the health system
- Generate research questions on causes, clinical outcomes of stroke, translational research on stroke
- Facilitate development of guidelines on management of stroke and quality of care indicators for stroke in India
- Support programmatic and policy interventions within National Program for Prevention and Control of Cancer, Diabetes, CVD and Stroke (NPCDCS) for strengthening access to care.
- Host studies (observational and experimental) within the registry network.
- Data has great value for national and international comparisons
Stroke data Transmission by HBSRs and PBSRSs

HBSR
Hospital based stroke registry (HBSR) is a hospital that collects data using a standardized core form on persons with stroke who have received treatment in the respective hospital. The major objectives of a HBSR is to generate reliable data on pattern of stroke, pattern of care and treatment and survival outcomes of stroke among patients treated in the hospital.
Core form - Following information is collated:
- Patient identifying and demographic information
- Clinical symptoms and signs of stroke
- Details of diagnosis
- Imaging details
- Type of Stroke
- Risk factors and co-morbidities
- Details of treatment
- Complications during hospitalization
- Clinical outcomes during hospital stay
- Follow-up on day 28 and 3 months after onset of stroke
- Details of death
Pre-requisites for initiating Hospital Based Stroke Registry
-
The hospital should be a Medical college/ tertiary care centre/general hospital with neurology unit/ stroke care facilities.
The neurologist or clinician (later may be Principal Investigator-PI) who is interested in starting a stroke registry should initiate the process with the concurrence and necessary permission of the Head of the institution.
- A sensitization meeting or discussion may be conducted within the hospital, so as to get cooperation from all the departments in which stroke patients are being diagnosed/ treated in the institution. Doctors of other Departments who are interested and willing to work on establishing stroke registry may be identified.
- A dedicated staff and exclusive space for functioning of registry should be available
- A computer with good internet connectivity for data entry, transmission and data management is necessary
Steps to Register as HBSR:
The prospective HBSR has to register by completing the HBSR registration form (pdf for reference) in the website www.stroke.ncdirindia.org/
The signed Registration Form with Signature and Seal of Head of Institution may be sent by post to ICMR-NCDIR. The Head of the Institution may identify suitable Principal Investigator (PI) and Co-PIs for this project.
The application shall be scrutinized at ICMR-NCDIR to understand the feasibility of establishing HBSR in the participating hospital and accordingly the next steps are initiated.
List of Activities for commencement of Hospital Based Stroke Registries (HBSRs)
- An agreement (MoU) between participating hospital and ICMR- NCDIR would be finalized. Certificate of Ethical Clearance from the Institutional Ethics Committee should be obtained from the participating hospital.
- After receiving registration form, MoU, IEC certificate, NCDIR would provide the necessary training on HBSR, and credentials to access and transmit data using online HBSR software free of cost
- Materials such as hardcopy core form, procedure manual will be provided by NCDIR
- HBSR Inclusion criteria is all first ever and recurrent stroke cases of age ≥18 years presenting within a month of onset of stroke to the reporting hospital.
- The hospital should be able to abstract data from all the cases admitted since 01 January of the current calendar year. IEC approval for retrospective data collection shall be obtained by the hospital if necessary.
- An inter–departmental meeting should be held at periodic intervals as collection of cases from all the departments in the hospital, hence inter department cooperation is essential. The primary departments concerned are: a. Neurology Departments (Surgical, Medical etc.,) b. Non-Neurology Departments (Medicine, Emergency, Radiology, General Surgery, Obs and Gynaecology, Physiotherapy, Paediatrics, Rehabilitation etc.)
- Dedicated staff for visiting departments and collection of data along with computer and internet connectivity to transmit the data
Data management
Data are recorded in the core form supported by detailed procedure manuals and transmitted to the ICMR-NCDIR through a secure, web-based software system. Data transmitted online using authenticated login credentials are encrypted, and data security and confidentiality of data are maintained. The hospital PI and Co-PIs and the concerned staff should participate in the meetings /workshops /training programs and present the progress of work. The PI shall be responsible for the data management and quality.
NCDIR team coordination, Data management, analysis
- The NCDIR team coordinating unit will support individual hospital in establishing the HBSR with inputs in technology, epidemiology and field work.
- Meetings and workshops for the HBSR hospital on purpose of the HBSR, the core form, standardization of the methodology of collecting and transmitting data, and planning out the data collection process will be conducted.
- The NCDIR team has developed online data with in-built quality checks, and will provide technical support for use of software, free of cost.
Disclaimer
ICMR-NCDIR reserves the right to update or change information provided at any time. There are no financial assistance provisions available with ICMR –NCDIR for joining the project. The hospitals who are interested and willing to participate in the HBSR program of the NSRP may read the guidelines above and decide to register. The guidelines provided are only for representative purpose and should not be used for legal purposes For any further information or query or clarification, Please write to
Director,
National Centre for Disease Informatics and Research,
Indian Council of Medical Research, Department of Health Research, Ministry of Health and Family Welfare, Government of India,
Nirmal Bhawan-ICMR Complex (II Floor), Poojanahalli, N.H–7, Kannamangala Post,
Bengaluru–562 110 (India),
Email: ncdir@ncdirindia.org